valence electron configuration
Web Valence electrons located on an atoms outermost shell affect how an atom will behave with other atoms. 410 və-SEP-ər is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms.
 |
| Answered What Is The Valence Electron Configurati Inorganic Chemistry |
Web An atoms electron configuration describes the way its electrons fill sublevels when the atom is in its ground state.
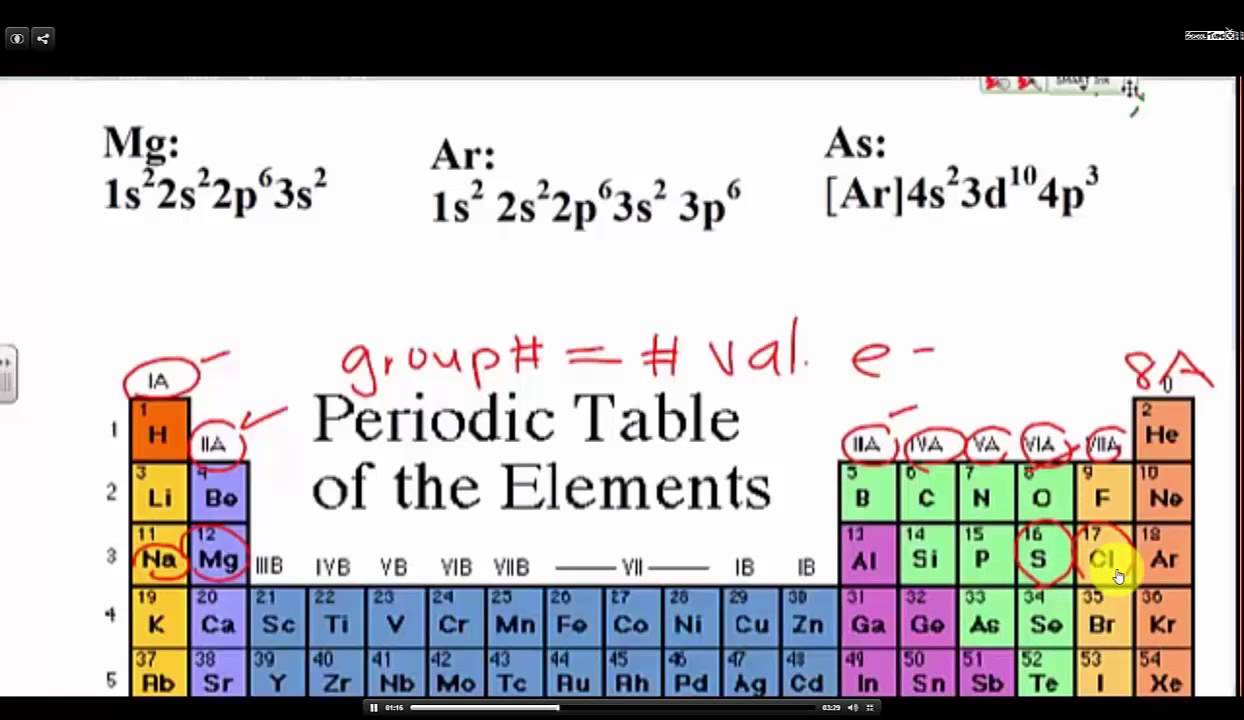
. This electron configuration shows that the antimony ionSb 5 has four shells and the last shell has eighteen electrons and it achieves a stable electron configuration. Therefore the valence electrons of radium are two. Web Electron configuration- Electron configuration is the arrangement of electrons in atomic orbitalsIt shows the electrons in numbers It doesnt show the details on the spin of electrons like the orbital diagram. Web Electron configuration- Electron configuration is the arrangement of electrons in atomic orbitalsIt shows the electrons in numbers It doesnt show the details on the spin of electrons like the orbital diagram.
Web The electron configuration of sulfide ionS 2- is 1s 2 2s 2 2p 6 3s 2 3p 6. Web Electron configuration- Electron configuration is the arrangement of electrons in atomic orbitalsIt shows the electrons in numbers It doesnt show the details on the spin of electrons like the orbital diagram. Web The electron configuration of antimony ionSb 5 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. Find out more.
The short electron configuration of oxygen is 2s 2 2p 4. This electron configuration of strontium ion shows that the strontium ionSr 2 has acquired the electron configuration of krypton and it achieves a. Electron configuration through orbit Bohr principle Electron configuration through orbital Aufbau principle Electron configuration through orbitals follows different principles. Web Overview Electron configuration.
Web The configuration of these electrons follows from the principles of quantum mechanics. Web The radium electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6 7s2. Valence Electron Definition in Chemistry. They live in energy levels or shells which are varyi.
The elements that. The electron configuration of silicon in excited state is Si14 1s 2 2s 2 2p 6 3s 1 3p x 1 3p y 1 3p z 1. Atoms seek the most stable electron configuration so sublevels are half-filled or fully-filled whenever possible. In the periodic table the elements are listed in order of increasing atomic number Z.
Therefore the calcium full electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2. So the next six electrons enter the 2p orbital and the remaining one electron enters the 3s orbital. Web Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons. Learn more about a valence electron including its configuration and examples.
Noble Gas Core Definition. Principal Energy Level Definition. Web It has only one valence electron present in the outermost shell which it readily loses to attain the stable noble gas electronic configuration of Neon left 28 right Meanwhile an atom of a Group 17 element such as chlorine has seven valence electrons in its outermost shell. The electron configuration of silicon shows that the last shell of silicon has four electrons.
Therefore the oxygen full electron configuration will be 1s 2 2s 2 2p 4. Web The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell giving it the same electronic configuration as a noble gasThe rule is especially applicable to carbon nitrogen oxygen and the halogens. Valence electrons- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic. Valence electrons- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic.
Web Note that these electron configurations are given for neutral atoms in the gas phase which are not the same as the electron configurations for the same atoms in chemical environments. The electron configuration of sulfide ion shows that the sulfide ionS 2- has acquired the electron configuration of argon and it achieves a stable. Web The p-orbital can have a maximum of six electrons. Web Electron Configuration Diagrams Properties of Matter Chemistry FuseSchoolLearn the basics about Drawing electron configuration diagrams.
The electrons that determine valence how an atom reacts chemically are those with the highest energy. In many cases multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to. Thus the number of valence electrons that it may have. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes.
Although more generally the rule is. Web The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The short electron configuration of sodium is 3s 1. This electron configuration shows that sulfide ionS 2- has three shells and the 3rd shell has eight electrons.
The total number of electrons in calcium is twenty and its symbol is Ca. The radium electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6 7s2. When writing an electron configuration you have to. So the remaining four electrons enter the 2p orbital.
Therefore the sodium full electron configuration will be 1s 2 2s 2 2p 6 3s 1. Web Valence shell electron pair repulsion VSEPR theory ˈ v ɛ s p ər v ə ˈ s ɛ p ər VESP-ər. It is also named the Gillespie-Nyholm theory after its two main developers Ronald Gillespie and. Web The electron configuration of strontium ionSr 2 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6.
The number of electrons in each elements electron shells particularly the outermost valence shell is the primary factor in determining its chemical bonding behavior. Web The total number of electrons in a valence shell is called a valence electron. The antimony atom exhibit -3 3 5 oxidation states. Therefore the valence electrons of silicon are four.
For example Aufbau principle Hunds principle and Paulis exclusion principle. Web Therefore the calcium full electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2. Valence electrons- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital.
Radium is the 88th element in the periodic table. Web The electron configuration of an aluminumAl atom can be done in two ways. Web In atomic theory and quantum mechanics an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This valence electron participates in the formation of bonds with atoms of other elements.
This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleusThe term atomic orbital may also refer to the physical region or space. Web The p-orbital can have a maximum of six electrons. For a main-group element the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. When writing an electron configuration you have to write serially.
The formalism has been incorporated into the two major models used.
 |
| Solved Complete The Valence Electron Configurations For Each Chegg Com |
 |
| Chemistry 101 Valence And Core Electrons Youtube |
 |
| Electron Configuration Anomalies Villanova College Chemistry Blog |
 |
| What Is The General Valence Shell Electronic Configuration Of Group 14 Elements Youtube |
 |
| Solved Complete The Valence Electron Configurations For Each Molecule Below Compound Valence Electron Configuration 62s 2 6 28 2 T2p 2 G2s 6 2s Tzp Nz 62s 6 2s T2p 62p 02 02s 0 2s |
Posting Komentar untuk "valence electron configuration"